Magnetic Resonance Widened Diagnostic Criteria for Hepatocellular Carcinoma
Copyright
© 2017 Upon acceptance of an article for publication in Hellenic Journal of Radiology, authors transfer copyright to the Hellenic Radiological Society but they retain the intellectual property rights including research data.
Introduction
In cirrhosis, hepatocarcinogenesis is a complex and multistep process due to accumulation of genetic and epigenetic changes, creating a microenvironment replete with carcinogens in the chronically diseased liver, leading to the development of hepatocellular carcinoma (HCC) from dysplastic liver nodules. An appropriate system of diagnosis and stratification is currently based on the patient’s condition, the Child-Pugh score, the carcinogenesis and natural history of the tumour, the available diagnostic criteria and the defined prognostic phenotypes. All of them establish categories of staging that correspond with certain treatment options. In this setting, standardised diagnostic and reporting systems improve the quality of communication between radiologists, clinicians and patients.
It is agreed that HCC in cirrhotic patients allows a noninvasive imaging diagnosis without histologic confirmation with nearly perfect specificity and positive predictive values. There are several guidelines for the noninvasive diagnosis of HCC on the cirrhotic liver, such as the European Association for the Study of the Liver (EASL), the American Association for the Study of Liver Diseases (AASLD) and the Liver Imaging Reporting and Data System (LI-RADS) [1-3]. They use different imaging criteria based on the size of the lesion (larger diameter greater than 1 cm) and enhancement after administration of intravenous contrast medium (hypervascular in the arterial phase with a wash-out pattern in the portal and delayed phases after extracellular contrast agent administration). In addition, the EASL guidelines include origin of the performed study (if it is a center of excellence) and the diagnostic technique (CT or MR imaging). The newly developed LI-RADS criteria provide a gradation of diagnostic probability of HCC based on the behaviour of the lesion in the arterial phase, the presence of contrast wash-out in the portal phase, the existence of a capsule appearance, and the evidence of tumour growth in serial imaging studies. Recently, the EASL-AASLD recommendations have been validated in a large multicentre study [4], reinforcing also the role of contrast-enhanced ultrasound (CEUS) in the non-invasive diagnosis of HCC.
However, the published comparative analyses of these guidelines show some discrepancies in the radiological diagnosis of HCC [5]. According to a meta-analysis, the overall per-lesion sensitivity of MR imaging was significantly higher than that of multidetector CT (80% vs 68%, p=0.0023), both sensitivities being significantly lower for tumours smaller than 1 cm [6]. In a different meta-analysis, pooled direct comparison of test performance in the detection of HCC showed similar overall sensitivities of 71% and 81% for CT and MRI, respectively [7]. On the other hand, according to the AASLD revised guidelines, the specificity in the detection of small HCC, less than 2 cm, decreases to 44% with both techniques [8]. Furthermore, using the LI-RADS criteria, the HCC diagnosis cases were 0% for LI-RADS-1 (definitely benign), 25% for LI-RADS-2 (probably benign), 69% for LI-RADS-3 (intermediate probability), 96% for LI-RADS- 4 (probably HCC) and 98% for LI-RADS-5 (definitely HCC) [9]. However, although the appearance of the capsule and the establishment of lesion growth, are extremely relevant for the diagnosis of HCC, these criteria are only explicitly contemplated in the LI-RADS classification. In addition, the evaluation of growth of the lesion in the follow-up of these patients is a parameter that can be misleading due to aspects such as extremely slow growing in small lesions, variability that may exist between readers, the use of different imaging techniques or the absence of previous examinations available at the time of the report.
The aim of our study is to assess the reliability of a comprehensive radiological diagnostic guideline for the non-invasive MR imaging identification of HCC in cirrhotic livers. The guideline developed in our center considers the added value of several different ancillary minor criteria and does not include growth or size in order to minimise difficulties and biases. This criterion will be compared to the most widely used and established guidelines (EASL-AASL, LI-RADS).
Materials and Methods
2.1 Design and Population
This descriptive observational retrospective study includes 77 consecutive patients diagnosed with HCC greater than 5 mm in a 24 months’ period (from January 2012 to February 2014). All cases had liver cirrhosis except for two with chronic hepatitis C viral infection. Eleven patients were finally excluded because of lack of verification. The remaining 66 cases had confirmation obtained by pathologic analysis (36 tumours, 54.5%, including the 2 HCC cases smaller than 1 cm), or by the combination of all these criteria (30 cases, 45.5%): standard imaging, analytical (alpha-fetoprotein greater than 20 ng/ml) and follow-up (malignant behaviour) [1]. From pathology reports, there were 15 cases without defined pathologic grade, 5 HCC grade I, 6 HCC grade II, 5 HCC grade III, 2 fibrolamellar HCC, and 3 bi-phenotypic HCC-CCC (hepatocellular-cholangiocellular carcinoma). The fibrolamellar tumours were present in patients with viral chronic hepatitis, aged 28 and 51 years. For the groups, mean age was 62 years (range, 23 to 83 years). Most patients (57 cases, 86.4%) were men, with a mean age of 62 years; the remaining 9 patients were women (13.6%) with a mean age of 60 years.
MR was performed on different 1.5 and 3T scanners. Standard gradient echo chemical shift T1-weighted, turbo-spin echo T2-weighted, diffusion-weighted with high b-values (800-1,000 s/mm²) and dynamic spoiled gradient echo T1-weighted sequences (late arterial, portal and equilibrium phases after 0.1 mmol/Kg body weight of gadobenate dimeglumine –MultiHance, Bracco, Italy at 2 ml/s rate injection) were used. Hepatobiliary phase images were not obtained in this study because this is the gadolinium-based contrast agent available in our center and in the first patients of the study this phase was not included in our protocol.
All cases were evaluated by two radiologists (5 and 8 years of experience in abdominal MR) in consensus, using the EASL/AASLD, LI-RADS and our own multivariate classification (Valencia multivariate, VLC-MV). The comparison was made between the percentage of patients correctly diagnosed according to the diagnostic criteria of the different classifications (EASL/AASLD, LI-RADS 2014 and VLC-MV), and the gold-standard, considered as the pathological confirmation or the presence of established diagnostic criteria including imaging, analytical and follow-up data.
All classifications considered the typical vascular behaviour as homogeneous enhancement in the late arterial phase (20 seconds delay time after the initial enhancement of the lower thoracic aorta) and wash-out in the portal phase (50 seconds after initial aorta enhancement) and/or in the equilibrium phase (at least 120 seconds after initial contrast enhancement).
In the EASL/AASLD criteria for excellence centers, the following parameters were considered diagnostic: lesion diameter greater than 1 cm, enhancement in the arterial phase and portal phase wash-out, and the possibility of a positive diagnosis both with CT and MRI [1, 2]. LI-RADS criteria (2014) consider either hypo or isoenhancing or hyperenhancing lesions in the arterial phase, then classify them according to their size, the presence of none, one or two imaging criteria (wash-out in the portal phase and pseudocapsule) and threshold growth [3].
The VLC-MV criteria have been developed in our center and have been in use for the last 15 years in the diagnosis and management of HCC with MR imaging [Table 1]. For the diagnosis of HCC using the VLC-MV classification, the lesion has to present at least one vascular major criterion (hyperenhancement in the arterial phase and/or wash-out in the portal phase) plus at least two minor criteria (pseudocapsule, fatty metamorphosis, mosaic pattern, signal changes on T1-weighted and T2-weighted sequences, restricted diffusion, and vascular invasion). Fatty metamorphosis was evaluated using fat suppression T2-weighted and T1-weighted in/out sequences. Restriction in difussion-weighted imaging was assessed as a hyperintensity in high b value imaging (800 or 1,000) corresponding to a hypointensity in the ADC map. As this was planned as a single reading accuracy criterion, neither tumour growth (as control studies were not always present) nor hepatocellular phase data (as hepatobiliary images were not acquired in this series) were considered in this analysis.
Table 1
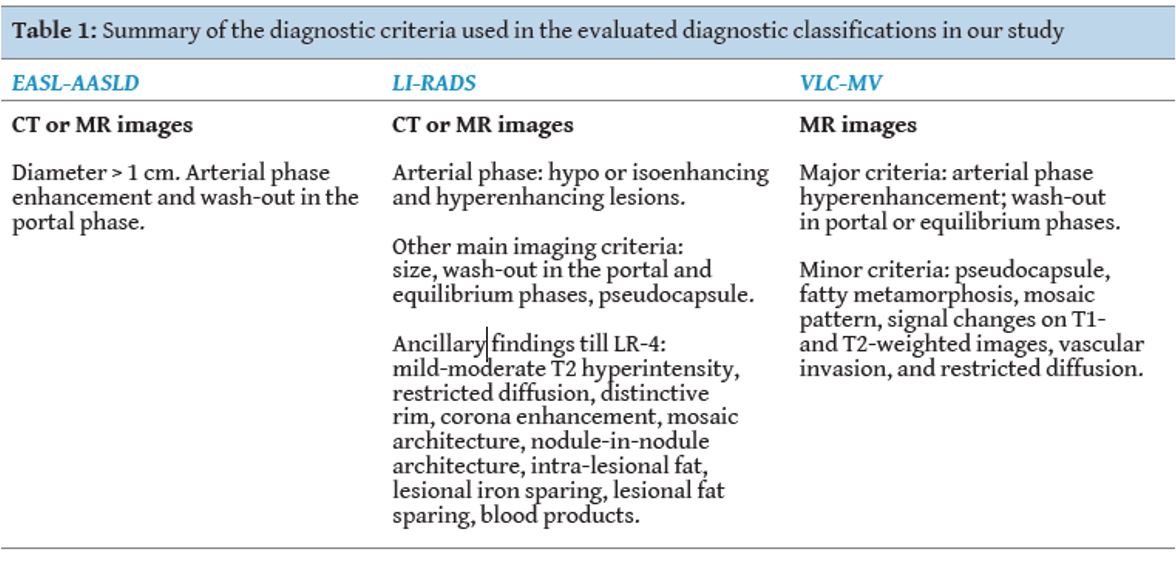
[Figure ID: ]
Results
Mean HCC size was 38.6 ± 26 mm (standard deviation), with a range from 7 to 120 mm. There were 6 hypovascular tumours (9.1%) and 4 (6.1%) others without wash-out. No case presented with hypovascularity and no wash-out. Regarding minor ancillary criteria, pseudocapsule was observed in 57 cases (86.4%), fatty metamorphosis in 11 (16.7%), T1-/T2 slight signal changes in 18 (27.3%), mosaic pattern in 5 (7.6%), and diffusion hyperintensity in 45 (68.2%). Vascular invasion was observed in 6 cases (9.1%).
All the evaluated guidelines had a large positive predictive value, over 93%. The VLC-MV was the most accurate classification (98.5% of positive predictive value, 65/66), followed by LI-RADS (84.8% for LI-RADS-5, 56/66; 6 of them with venous vascular invasion LI-RADS-5V). The typical appearance, considered as the EASL/AASLD evaluated jointly, had a slightly worse positive predictive value (80.3%, 53/66). The differences between VLC-MV with LI-RADS and EASL-AASLD were statistically significant (Chi-Squared test, p=0.04 and 0.02).
There were 11 patients properly diagnosed with VLC-MV but undiagnosed in the EASL-AASLD classifications. Two of them had a diameter smaller than 10 mm, being excluded because of size in the EASL-AASLD classifications. The other 9 HCCs did not show either arterial enhancement or wash-out in the portal phase. These 11 patients were classified as LI-RADS-4 in 9 cases and LI-RADS 5 in 2 cases. All of them had at least 1 major and 2 or more minor criteria, fulfilling the VLC-MV guideline. The most common ancillary criteria were hyperintensity on T2-weighted images, pseudocapsule and restriction on diffusion-weighted images. These cases were proven by pathology (Fig. 1 and 2). The case failed by the VLC-MV analysis was a fibrolamellar HCC.
Figure 1
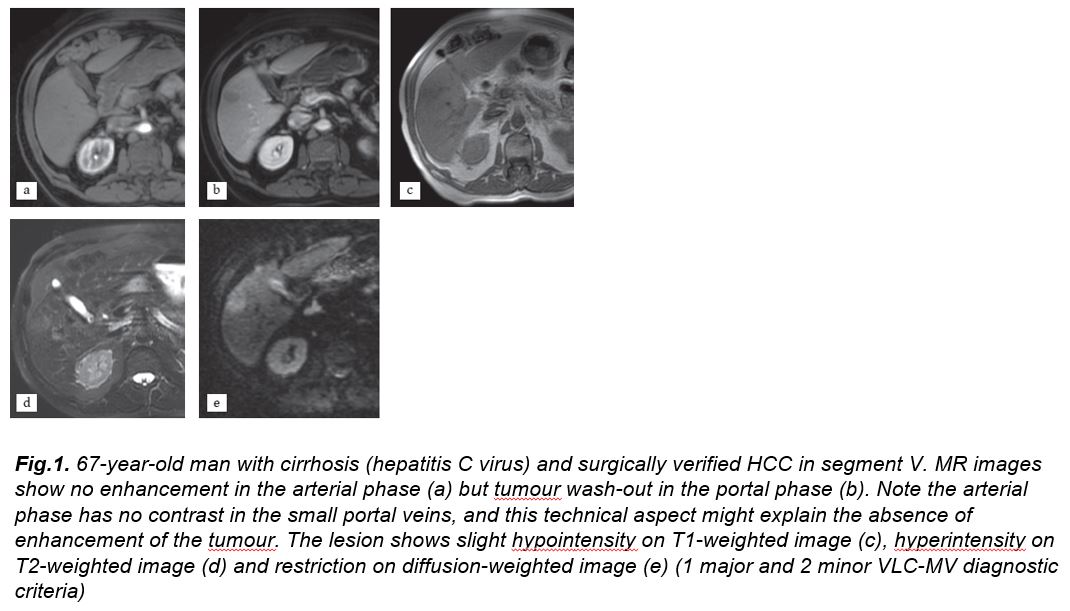
[Figure ID: ]
Figure 2
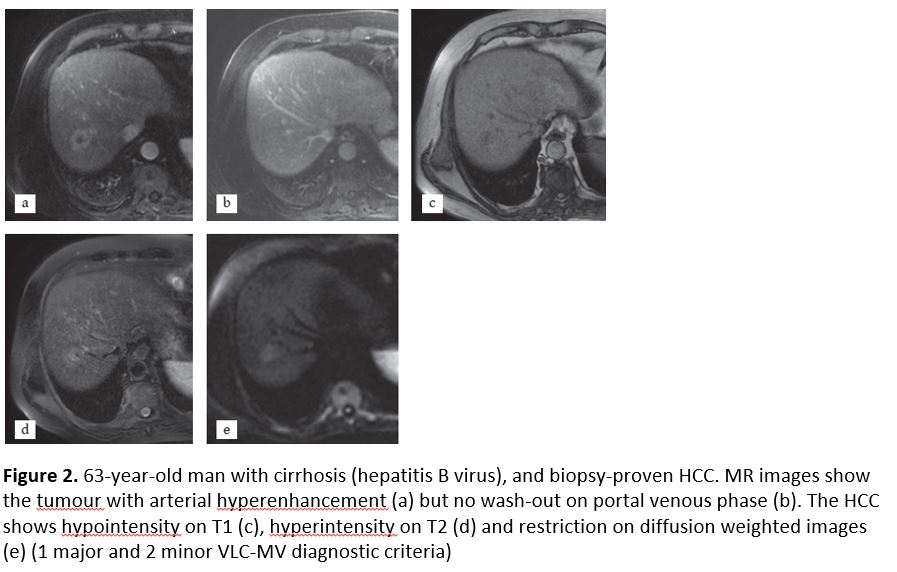
[Figure ID: ]
Discussion
According to clinical guidelines, the HCC diagnosis is based on the development of unpaired arteries and decreased portal blood supply during the hepatocarcinogenesis process, from regeneration node to the HCC [10, 11]. CT but mainly MR dynamic studies with extracellular contrast agents allow the non-invasive characterisation of HCC based on the arterial hyperenhancement and portal-equilibrium phase wash-out.
Surprisingly, additional information routinely evaluated in hepatic MRI studies are not considered in most guidelines. These provide data on the lesion structure, composition and cellularity [12]. The ancillary signs are also characteristics of HCC and include the following: i) diffusion restriction caused by hypercellularity; ii) presence of intratumoural fat; iii) moderate signal signal changes on T1- and/or T2-weighted images compared to the surrounding liver; iv) inner mosaic pattern with homogeneous areas separate by thin septa; v) presence of pseudocapsule with a delayed enhancement; vi) absence of ferric content in the presence of liver iron overload; vii) vascular tumoural invasion; and viii) the nodule-within-nodule appearance [13]. In addition, growth of the nodule also supports the diagnosis of malignancy in a liver lesion. All these criteria should be recognised in order to increase the radiologists’ diagnostic confidence.
Special mention should be made to small nodules detected on ultrasound screening in cirrhotic patients. In these cases, the sensitivity and specificity of imaging techniques such as CT and MRI to confirm or rule out the presence of HCC are considered lower than in larger nodules [14, 15], especially when CT is used [16]. These small nodules, either high-grade dysplastic or early HCC, may not exhibit the typical vascular behaviour of established HCC (arterial phase hyper enhancement and portal-venous phase wash-out) due to the moderate development of unpaired arterial vascularisation with preservation of the portal flow. Therefore, the ability of imaging to characterise them is generally weak, but possible. Pahwa et al., in a cohort of 156 risk patients with new-onset hepatic nodules analysed with the AASLD, obtained 21% of histologically proven small HCC which could not be correctly diagnosed according to these criteria [17]. However, alternatively, it can be argued that even in these small nodules imaging can reach the diagnosis in 79% of cases without biopsy.
Recently, the American College of Radiology developed the LI-RADS system for stratifying the probability of HCC, in which, in addition to the vascularisation of the nodule, it also considers other characteristics such as growth rate and the contrast uptake pattern by the capsule [3]. Our VLC-MV approach fully agrees with the use of these minor criteria to improve diagnostic accuracy.
Several publications have evaluated the increased sensitivity, specificity and diagnostic accuracy incorporating the diffusion weighted sequences and the ancillary diagnostic findings such as signal hyperintensity in T2-weighted sequences, or the presence of fat or iron. The use of new hepatospecific contrast agents also impacted the diagnostic accuracy of imaging. The hepatocarcinogenesis process reveals an alteration in the pharmacokinetics of currently available hepatospecific contrast agents, such as gadoxetic acid and gadobenate dimeglumine, due to the loss of certain hepatocyte membrane transporters in their dedifferentiation cascade, specifically with the gradual decrease in the expression of OATP8 in these cells. As a consequence, most HCCs are hypointense in the hepatocellular phase [18]. The KLCSG-NCC and JSH guidelines contemplate the nodule signal with respect to the rest of the liver parenchyma in the hepatocellular phase, and the latter also considers the behaviour on sonography with Sonazoid® contrast [19, 20]. We did not consider these images as most cases were evaluated without this hepatobiliary phase.
Park et al. achieved a greater sensitivity and diagnostic accuracy in the detection and characterisation of hepatic nodules smaller than 2 cm with the combination of the diffusion sequence and the hepatocellular phase after the administration of gadoxetic acid. The improvement was enhanced when the two sequences were used in combination rather than separately [21]. Rimola et al. did not find substantial differences in diagnostic accuracy when fat metamorphosis or T2 hyperintensity are considered as ancillary criteria in the diagnosis of HCC in nodules smaller or equal to 2 cm, although the presence of pseudocapsule was highly specific in lesions with typical vascular HCC behaviour [22].
In our series, the VLC-MV guideline presented a high accuracy in the diagnosis of HCC, higher than the other classifications assessed in this study. According to the results of this study there were 11 patients that the EASL-AASLD guidelines could not diagnose. This is due, in our opinion, to two fundamental reasons: the first is that in the EASL-AASLD classifications the two vascular criteria, arterial enhancement and portal wash-out must be met in order to establish the definitive diagnosis of HCC. In our classification, only one of these vascular criteria can be enough to establish the diagnosis of HCC in the presence of two minor criteria. The accuracy of the VLC-MV classification is greater because it does not discriminate lesions according to their size, improving thus the positive predictive value. In this study, two of the lesions were less than 1 cm and therefore were excluded from the EASL-AASLD classifications. In our classification, if the lesions meet the major and minor criteria they are not excluded by size, thus increasing the diagnostic performance of imaging.
The only case that our classification did not accurately diagnose was a fibrolamellar hepatocarcinoma, because these tumours occur in young patients without cirrhosis but can also complicate chronic hepatitis viral infection. The imaging behaviour of these tumours is different from the typical HCCs that occur in cirrhotic livers, and the criteria used might not be valid.
The minor criteria most often found in our study were contrast uptake pattern of the capsule, hyperintensity in T2-weighted sequences and restriction in the diffusion sequence. As previously mentioned [22], the presence of pseudocapsule is treated as a highly specific finding.
Limitations of this study have to be considered. As only HCC cases were included in the study, and other lesions can be encountered in patients with chronic liver diseases mimicking HCC, specificity could not be evaluated. The behaviour of a lesion in the hepatobiliary phase is an important diagnostic criterion that has not been included in this series as the used MR protocol did not include the delayed hepatobiliary phase in most cases. The observed improvement in accuracy, even without the use of the hepatobiliary phase information or availability of previous studies to demonstrate lesion growth, reinforced the value of the VLC-MV criteria and the need to evaluate further improvements with these added criteria. Therefore, we have demonstrated the utility of integrating some of these ancillary findings in the diagnostic process even when the typical vascular criteria of HCC have not been demonstrated. Even more, as we evaluated the radiological findings in consensus between two radiologists, interobserver agreement could not be demonstrated. Another limitation is that the overall accuracy of the VLC-MV criteria should be evaluated in surgical series that include HCC and other non-HCC lesions. In future studies, the specificity of the VLC-MV classification will be assessed with an exclusively pathologic reference pattern, such as liver explants due to HCC, including all different pathologic lesions in a prospective manner. This new study will prove useful to evaluate false positive (patients diagnosed with HCC following this classification but without pathological evidence of malignancy or having other pathologic diagnosis) and false negative (patients having been diagnosed with other non-HCC tumour).
In conclusion, the MR imaging VLC-MV classification is an accurate method for the non-invasive diagnosis of HCC, having the advantage of not requiring the evaluation of growth and not being limited by lesion size. R
Conflict of interest:
The authors declared no conflicts of interest.
References
1. EASL-EORTC Clinical practice guidelines: Management of hepatocellular carcinoma. European association for the study of the liver, European Organization of Research and Treatment of Cancer. J Hepatol 2012; 56: 908-943.
2. Bruix J, Sherman M. American Association for the study of liver diseases (AASLD). Management of hepatocellular carcinoma: An update. Hepatology 2011; 53: 1020-1022.
3. Liver imaging reporting and data system (LI-RADS) 2014. American College of Radiology. http: https:// nrdr.acr.org/lirads/.
4. Aubé C, Oberti F, Lonjon J, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int 2017; 37(10): 1515-1525.
5. Cruite I, Tang A, Sirlin CB. Imaging-based diagnostic systems for hepatocellular carcinoma. AJR Am J Roentgenol 2013; 201: 41-55.
6. Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015; 275: 97-109.
7. Chou R, Cuevas C, Fu R, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Ann Intern Med 2015; 162(10): 697-711.
8. Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging technique in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010; 59: 638-644.
9. Darnell A, Forner A, Rimola J, et al. Liver imaging reporting and data system with MRimaging: Evaluation in nodules 20 mm or smaller detected in cirrhosis at screening US. Radiology 2015; 275: 698-707.
10. Park YN, Kim MJ. Hepatocarcinogenesis: Imaging-pathologic correlation. Abdom Imaging 2011; 36: 232–243.
11. Kudo M. Multistep human hepatocarcinogenesis: Correlation of imaging with pathology. J Gastroenterol 2009; 44 Suppl 19: 112-118.
12. Lee JM, Yoon JH, Kim KW. Diagnosis of hepatocellular carcinoma: Newer radiological tools. Semin Oncol 2012; 39: 399-409.
13. Hennedige T, Venkatesh SK. Advances in computed tomography and magnetic resonance imaging of hepatocellular carcinoma. World J Gastroenterol 2016; 22: 205-220.
14. Quaia E, De Paoli L, Angileri R, et al. Evidence of diagnostic enhancement pattern in hepatocellular carcinoma nodules <2 cm according to the AASLD/EASL revised criteria. Abdom Imaging 2013; 38: 1245-1253.
15. Kierans AS, Kang SK, Rosenkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: A meta- analysis. Radiology 2016; 278: 82-94.
16. Sersté T, Barrau V, Ozenne V, et al. Accuracy and disagreement of computed tomography and magnetic resonance imaging for the diagnosis of small hepatocellular carcinoma and dysplastic nodules: Role of biopsy. Hepatology 2012; 55: 800-806.
17. Pahwa A, Beckett K, Channual S, et al. Efficacy of the American Association for the study of liver disease and Barcelona criteria for the diagnosis of hepatocellular carcinoma. Abdom Imaging 2014; 39: 753-760.
18. Kitao A, Matsui O, Yoneda N, et al. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: Correlation with gadoxetic acid enhanced MR imaging. Eur Radiol 2011; 21: 2056-2066.
19. Yoon JH, Park JW, Lee JM. Noninvasive diagnosis of hepatocellular carcinoma: Elaboration on Korean liver cancer study Group-National Cancer Center Korea practice guidelines compared with other guidelines and remaining issues. Korean J Radiol 2016; 17: 7-24.
20. JSH Consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 2014; 3: 458-468.
21. Park MJ, Kim YK, Lee MW, et al. Small hepatocellular carcinomas: Improved sensitivity by combining gadoxetic acid–enhanced and diffusion-weighted MR imaging patterns. Radiology 2012; 264: 761-770.
22. Rimola J, Forner A, Tremosini S, et al. Non-invasive diagnosis of hepatocellular carcinoma ≤ 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol 2012; 56: 1317-1323.
None
Refbacks
- There are currently no refbacks.







